Some device companies have already submitted their products and have received 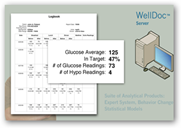 approval for FDA clearance such as WellDoc and ExpressMD. There are a few others I have reported on as well that have done the same; however there’s a lot of device software programs emerging, almost daily I think as I try to cover a lot of the products on this blog.
approval for FDA clearance such as WellDoc and ExpressMD. There are a few others I have reported on as well that have done the same; however there’s a lot of device software programs emerging, almost daily I think as I try to cover a lot of the products on this blog.
FDA Approves WellDoc Smartphone Mobile Diabetes Tool/Software Using Bluetooth And Automated Algorithmic Analysis Processes
ExpressMD Collaborates with Rotech Healthcare for TeleHealth Patient Monitoring–Electronic House Calls
Just today the FDA announced their FDA Track site to monitor their performance  and I think in their recall efforts its not done very well at all and is close to an “F” as they have no system by their own admittance.
and I think in their recall efforts its not done very well at all and is close to an “F” as they have no system by their own admittance.
I think internally the FDA should be experiencing a little of their own mobile technology as they don’t have any type of recall program and using cell phones would certainly give them some “first hand” experience. As a matter of fact it would help them tremendously in understanding how mobile health is working today and be able to pinpoint those mobile devices they feel need to come under an 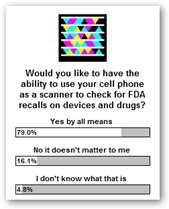 approval process for the FDA.
approval process for the FDA.
We have some bills going here but no action from the FDA since 2004.
Bill Seeks to Arm FDA with Full Drug Recall Power And More Over the Counter Regulatory Control
Technology and participation is for everyone today and we are really starting to see ![]() value with mobility so the FDA is the perfect candidate to participate I believe too. If you think there’s some merit to this, take time to vote and say yes if you would like to
value with mobility so the FDA is the perfect candidate to participate I believe too. If you think there’s some merit to this, take time to vote and say yes if you would like to  have the ability to find FDA recalls by scanning with your phone.
have the ability to find FDA recalls by scanning with your phone.
Here’s a company that is actually consulting and working with the FDA on nutrition, so what they are doing with mobile technology, so what’s up with the FDA and using mobile technology for recalls?
By the way I have sent my suggestion to the FDA a few times and received one generic website response so far. I think the FDA needs to get into the mobile technology ball game as again it will help make more sense in locating software they believe should have FDA approval. BD
The U.S. Food and Drug Administration is actively watching app stores for apps that it deems fill a medical purpose with an eye toward regulating them, according to Bradley Merrill Thompson, an attorney specializing in health care issues. Thompson said even smartphone apps require FDA approval, just like other medical “devices,” and the determination if an app requires federal approval is strictly the FDA’s call. Apps such as iStethoscope for the iPhone (featured today in the Telegraph) and Instant Heart Rate for Android may find themselves facing a regulatory process much like other medical devices such as glucose monitors, which could stymie innovation and put the kibosh on plans to use smartphones for health monitoring.

